On July 10, 2025, China National Center for Food Safety Risk Assessment (CFSA) issued 7 new food raw materials. Comments are welcomed before August 9, 2025. Details are as follows:
Gardenia oil
Name | Gardenia oil | |
Basic information | Source: fruits of Gardeniajasminoides J.Ellis | |
Production process | Made from fruits of Gardeniajasminoides J.Ellis, it is produced through processes such as screening, pressing, precipitation, and filtration. | |
Quality specifications | Characteristics | Pale yellow to brownish-yellow oily liquid. |
Fatty acid composition (as a percentage of total fatty acids) | ||
Oleic acid (C18:1), % | ≥20.0 | |
Linoleic acid (C18:2), % | ≥45.0 | |
Note | 1. The scope of use does not include infant foods. 2. Food safety indicators shall comply with the current national food safety standards of China for vegetable oils and fats. | |
XiangYu peony flower
Name | XiangYu peony flower |
Basic information | Source: flowers of Paeoniasuf ruticosa ‘Xiang Yu’ |
Production process | Made from flowers of Paeoniasuf ruticosa ‘Xiang Yu’, it is produced through processes such as picking and screening. |
Note | 1.Infants, pregnant women, and lactating women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations. 2.Food safety indicators shall comply with the current national food safety standards of China for vegetables. |
Alginate oligosaccharide
Name | Alginate oligosaccharide |
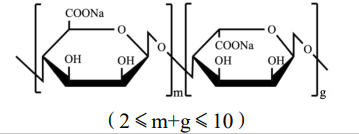
Basic information | Structure form:
|
Production process | Made from sodium alginate derived from Phaeophyta through processes including hydrochloric acid dissolution, high-temperature degradation, sodium hydroxide neutralization, decolorization, purification, concentration, and drying. |
Recommend intake | ≤4 g/day |
Note | 1. Infants, pregnant women, and lactating women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations. 2. Quality standards and food safety indicators refer to the appendix. |
Bifidobacterium longum subsp. Infantis YLGB-1496
Name | Bifidobacterium longum subsp. Infantis YLGB-1496 |
Note | 1. Approved for inclusion in the List of Microorganisms Permitted for Use in Infant Foods. 2. The food safety indicators shall comply with Microbial Preparations for Food Processing (GB 31639), and Cronobacter spp. must not be detected (/100 g). |
Peanut skins procyanidins
Name | Peanut skins procyanidins |
Basic information | Source: Seed coat derived from Arachis hypogaea L., a leguminous plant. |
Production process | Made from Seed coat derived from Arachis hypogaea L, it is produced through processes such as crushing, water extraction, concentration, filtration, sterilization, and drying. |
Recommend intake | ≤400 mg/day |
Note | 1.Scope of use and maximum dosage: - beverages: liquid beverages ≤50 mL packaging (8 g/kg), 51–500 mL packaging (0.8 g/kg), and solid beverages calculated based on the reconstituted liquid mass. - candy: 8 g/kg. 2. Infants, pregnant women, and lactating women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations and consumption limits. 3. Quality standards and food safety indicators refer to the appendix. |
Beta-lactoglobulin (fermentation)
Name | beta-lactoglobulin (fermentation) |
Production process | Produced from glucose, ammonium sulfate, ammonia water, and other raw materials through fermentation with Kluyveromyces lactis CJ-B1-P22, followed by separation, purification, and drying processes. |
Note | 1. Not suitable for individuals allergic to milk and dairy products. Labels and instructions must clearly indicate the unsuitable populations. 2. Food safety indicators shall comply with the current national food safety standards of China for vegetables. |
Mycoprotein from Fusarium venenatum
Name | Mycoprotein from Fusarium venenatum |
Production process | Produced using Fusarium venenatum strain A3/5 or TB01 as the production strain, through processes including fermentation, nucleic acid removal, inactivation, and filtration. |
Note | 1. Infants, pregnant women, and lactating women should avoid consumption. Labels and instructions must clearly indicate the unsuitable populations. 2. Quality standards and food safety indicators refer to the appendix. |
About CIRS Group
Established in 2012, the Food Business Division of CIRS Group has helped over 1,000 domestic and international food companies achieve one-stop compliance solutions. CIRS offers a full range of regulatory services covering novel food applications, synthetic biology-derived foods, U.S. GRAS notice, EU novel food application, health food registration, and food for special medical purposes (FSMP).
Our food services in China include but not limited to:
- China new food raw materials registration
- China new food additive registration
- China health food (dietary supplement) registration/filing
- China health food testing service
- China new food contact substance registration
- China food for special medical purpose (FSMP) registration
- China infant formula milk powder registration
If you need any assistance or have any questions, please get in touch with us via service@cirs-group.com.
Further information